Solid polymer electrolytes (SPEs) are still considered candidates to replace liquid electrolytes in high-safety and flexible lithium batteries due to their light weight, flexibility and shape versatility. However, the low ion transport efficiency of linear polymer
electrolytes remains the biggest challenge. The development of new polymer electrolytes is an effective way to improve ion transport capacity. Non-linear topologies such as hyperbranched, star, comb and brush have highly branching characteristics.
Compared to linear polymer electrolytes, topological polymer electrolytes have more functional groups, lower crystallisation, glass transition temperatures and better solubility. In particular, the large number of functional groups facilitates the dissociation
of lithium salts, thereby improving ionic conductivity. In addition, topological polymers have a strong design capability to meet the comprehensive performance requirements of SPEs. This paper reviews the research progress of topological polymer
electrolytes in recent years, and analyses the design ideas of topological polymer electrolytes. This paper also provides an outlook on the future development of spe. It is expected that the research in this paper will generate keen interest in the design of
advanced polymer electrolyte structures, provide insight into future research on novel spe, and promote the development of next-generation high-safety flexible energy storage devices.
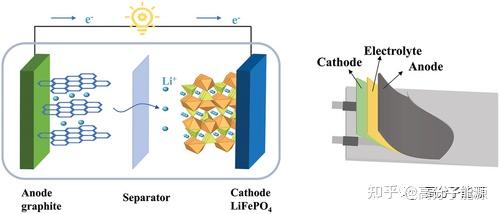
Structures of liquid Li-ion and flexible Li-ion batteries
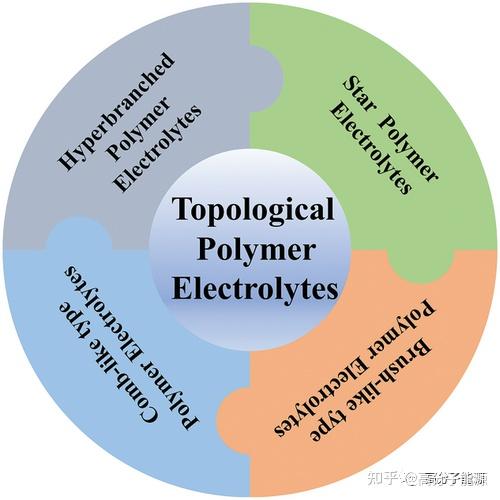
The types of topological polymer electrolytes are introduced.
Hyperbranched polymer electrolytes
It is well known that linear polymers such as PEO and epoxy polypropylene were the first spe polymer matrices to be used. Due to the high crystallinity of linear polymers, it is difficult to obtain high ionic conductivity in practical applications. To solve this
problem, topological systems have been developed in recent decades and have proven their ability to enhance the electrochemical properties of polymer electrolytes. Their specific topology limits the crystallisation of the polymer and facilitates carrier
migration. Hyperbranched polymers are the most typical representatives of topological polymers. They are a class of highly branched polymers with a 3D spherical structure that dates back to the late 19th century. In addition, its molecules exhibit
properties that are very different from their linear counterparts, such as low viscosity, good solubility and a large number of modifiable functional groups. In addition, the presence of a large number of branching points in the molecule can inhibit the
regular arrangement of the polymer chains, making them difficult to crystallise and thus improving the electrical conductivity of the polymer electrolyte. Hyperbranched polymers are therefore considered to be one of the most promising polymer
electrolyte substrates and have been extensively investigated.
Polyethers such as PEO are the earliest and most widely used polymer electrolyte substrates in previous studies, with good affinity for lithium salts and good compatibility with lithium metal. In addition, the large number of ether oxygen segments in
hyperbranched polyethers promotes ionic conduction. In this regard, Lee et al. investigated the relationship between ionic conductivity and branching degree as well as end-group functionality in hyperbranched structures. They demonstrated that the
unique hyperbranched poly(ethylene oxide) polymer (hbPEO) electrolyte had the ability to prevent crystallisation, increasing ionic conductivity by 6 × 10-5 S cm-1 under ambient conditions (Figure 3a). This new design strategy of using hyperbranched
structures to enhance ionic mobility provides a new idea for the preparation of SPEs with improved electrochemical properties.
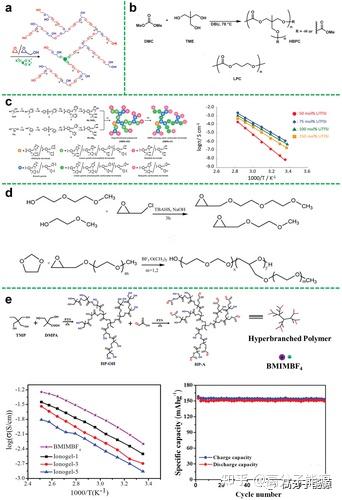
Schematic diagram of the synthesis of hyperbranched PEO. b) Synthesis of hyperbranched polycarbonate (HBPC) from dimethyl carbonate (DMC) and trimethylethane (TME). c) Synthesis pathway of diol-terminated hbpa (HBPA-OHs) and CC-terminated
hbpa (HBPA-CCs) from TMP and Gly under phosgene free conditions; synthesis of hbpa with different CC/ LiTFSI ratios for HBPA-CC-4/LiTFSI hybrid membranes prepared as a function of temperature. d) Synthetic route for copolymers. e) Schematic of the
synthesis of hyperbranched aliphatic polyesters (HP-A); Temperature dependence of the ionic conductivity of neat BMIMBF4 and ionic gels in the Arrhenius convention; Temperature dependence of the ionic conductivity of lithium/ionic gel/LiFePO4
batteries at room temperature; Temperature dependence of the ionic conductivity of lithium/ionic gel/ LiFePO4-type cells were charged and discharged at a rate of 0.1 C.
Polycarbonate backbone
In order to obtain high performance polymer electrolytes in a specific temperature range, careful design and effective selection of polymer functional groups and segment structures are required to effectively weaken anion-cation interactions. Amorphous
polymers with good segment flexibility are therefore an ideal class of polymer electrolyte matrix materials, and aliphatic polycarbonates are one of these. In particular, in addition to polyethers, polycarbonates with strong polar carbonate groups can also
be used for the synthesis of hyperbranched polymer electrolytes. In contrast to hyperbranched polyether electrolytes
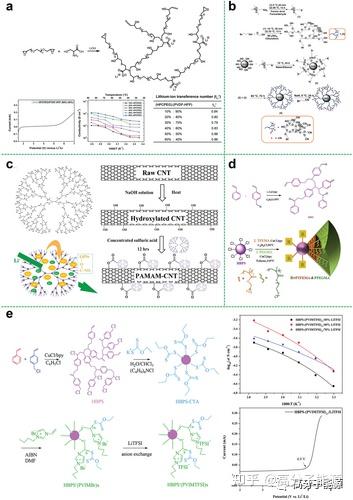
a) Synthesis of hyperbranched polyanionic HPCPEG; linear scanning voltammetry of SIPEs with 60% HPCPEG content at 25 °C; temperature dependence of ionic conductivity of HPCPEG/PVDF-HFP SIPEs lithium ion migration number of SIPEs with different
HPCPEG content at 25 °C. b) Synthesis of hyperbranched polyethylene glycol encapsulated silica nanoparticles. c) Chemical structure of PAMAM, schematic diagram of lithium polysulfide adsorption and Li+ transport via PAMAM, and schematic diagram of
PAMAM grafted onto carbon nanotubes via esterification. d) Synthetic route to fluorinated star-branched polymers. e) Synthetic route to hyperbranched star polymer ionic liquids; ionic conductivity measured at different temperatures for the VTF equation
(points) with fitted results (lines); HBPS -(PVIMTFSI)17/LiTFSI electrolytes with electrochemical windows
Star polymer electrolytes
Star polymers are an important class of polymers with topological structures. Star polymers can be formed by chemically bonding multiple linear polymer chains to a single core. With their spherically symmetrical topology, less chain entanglement and
lower crystallinity, star polymers can also be used as polymer electrolyte substrates. The topology of star-shaped polymers can effectively limit the crystallinity of the polymer matrix compared to conventional linear peo-based electrolytes. Whereas the
increase in segmental amorphous fraction and mobility facilitates ion transport, a large number of functional groups can be introduced into the arms or core of the star-shaped polymer matrix, facilitating the dissociation of the lithium salt. As a result, the
electrical conductivity of ions at room temperature can be greatly increased. In addition, the star-shaped polymer improves compatibility with the electrode surface to a certain extent due to its flexible polymeric support. Star polymers are therefore an
ideal electrolyte matrix material for solid state batteries.
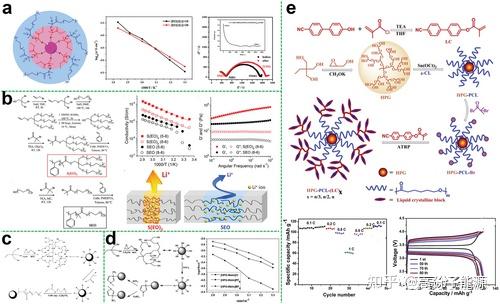
Star polymer electrolyte polyether cores hyperbranched polyethers as the core of star polymers can provide more conductive segments to dissolve more lithium salts and can be further modified with a large number of end groups to optimise
performance.
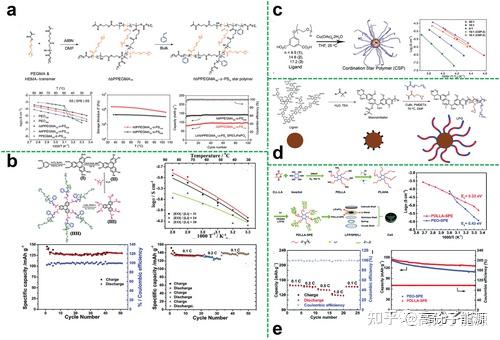
Hyperbranched polyethers as the core of a star polymer composite astropolymer electrolyte Sometimes, when an astropolymer electrolyte cannot be restructured to meet all the requirements of a high performance electrolyte, blending other organic or
inorganic components with the astropolymer electrolyte is an effective way to achieve a significant improvement in electrolyte performance.
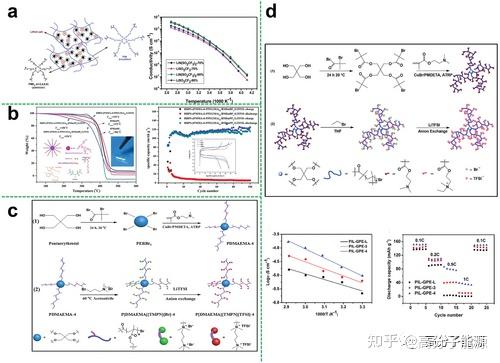
Plasticisers are ideal additives that can be used to modify electrolytes comb polymer electrolytes
To improve the ionic conductivity and mechanical properties of linear polymer electrolytes, grafting functionalised side chains onto the linear backbone can inhibit the crystallisation of the polymer and increase the mobility of the segments. Comb
polymers are a type of topological polymer which are formed by grafting on the main chain and can also be used as electrolyte substrates.
Comb polymer electrolytes based on different polymers
Depending on the polymer backbone, the reported comb polymer electrolytes are commonly found in the following categories
Comb copolymer electrolytes
Brush polymer electrolytes
Brush polymers or polymer brushes are special polymer structures formed by linking dense polymer molecules of a certain length to the surface of the substrate or to the polymer backbone. Polymer brushes have attracted a great deal of attention in the
field of solid polymer electrolytes due to their unique topology and functionality.
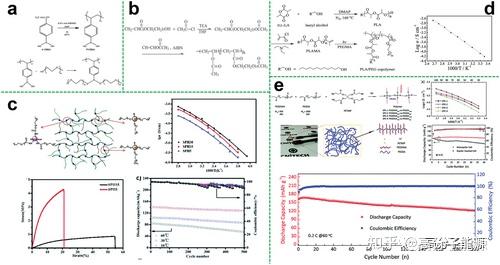
Brush copolymer electrolytes
Summary and outlook
The advent of spe promises to address the potential safety issues of liquid electrolytes. To date, significant efforts have been invested in these areas to further simultaneously improve the ionic conductivity and mechanical strength as well as the interfacial
compatibility of existing spe. The design of the molecular structure of polymer electrolyte matrices has received increasing attention as an approach to essentially solve the above problems. From this perspective, non-linear topological polymers such as
hyperbranched, star, comb and brush are the most promising solid polymer electrolyte materials due to their unique topology, amorphous shape, high solubility in common organic solvents, ease of film formation and the large number of functional
groups that can be further modified. Therefore, the use of various strategies such as structural design of polymer matrix or blending is expected to greatly improve the room temperature ionic conductivity, mechanical strength and even the overall
performance of topological polymer electrolytes. This paper reviews the research progress in topological polymer electrolytes in recent decades. While much progress has been made in this area, there are still some challenges that must be faced.
Specifically, with the increasing demand for high energy density memory devices, the development of electrolytes with high lithium storage performance and higher operating voltages has been seen as an urgent priority. At present, obtaining high
performance solid-state electrolytes for practical commercial applications remains a challenge. It is therefore important to develop new electrolytes that are in step with the times.
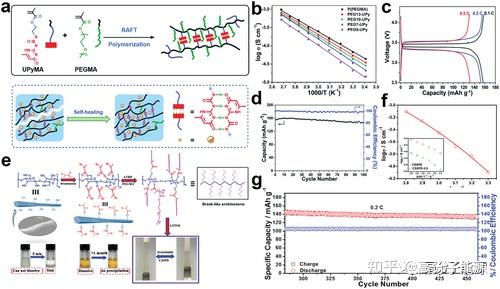
In this regard, future research on topological polymer electrolytes will focus on the following areas:1) designing new structures to achieve kinetic and thermodynamic stability at the electrolyte-electrode interface;2) combining experiments and theoretical
calculations to explore ion transport mechanisms;3) developing more attractive additives to integrate the performance of existing electrolytes;4) preparing new lithium salts to improve electrochemical performance;5) Exploring new techniques such as
optimising cell design structures to improve overall performance; 6) Reforming existing preparation methods or improving film formation processes to enable the application of topological polymer electrolytes; 7) Further developing new functional
electrolytes, including self-healing and highly stretchable polymer electrolytes, for flexible wearable devices. In addition, the above studies have practical implications for further applications of topological polymer electrolytes.
In summary, the excellent properties of topological polymer electrolytes make it possible to apply them in the next generation of high energy density lithium-ion batteries. Until today, an increasing number of researchers have done their best to promote
their progress. Theoretical predictions and practical operational improvements have provided new ideas for the development of SPEs. Although there is still a long way to go, research into the development of high-performance electrolytes will never stop.
As we expect, all-solid-state lithium-ion batteries with a variety of excellent properties will be developed and used in our daily lives.




































 401,Building A1,No.168,Changshan IndustrialZone Liulian Community,Pingdi Street,Shenzhen Guangdong Province,China
401,Building A1,No.168,Changshan IndustrialZone Liulian Community,Pingdi Street,Shenzhen Guangdong Province,China